The Radiation Mechanics of the Hydrogen Atom and the Structure of the Nucleus
 | |
| Author | James Carter |
|---|---|
| Published | 1998 |
| Publisher | James Carter |
| Pages | 86 |
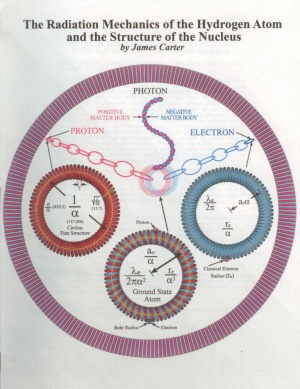
A mechanical model for the hydrogen atom is proposed in which the electron and proton are physically connected to one another by a series of torus shaped structures called circlons. Photons are produced at the interface when a circlon from the proton combines with a circlon from the electron. The atom's energy is contained within the stationary orbital motion of these components in which the circlons rotate on their axes but the electron does not revolve around the proton. A new orbital constant (Y) is proposed which is used to calculate the complete hydrogen radiation spectrum without the use Planck's constant (h). The Heisenberg Uncertainty Principle is used to define the size of the circlon rather than the uncertainty of the electron's position. At a deeper level, stationary orbital motion is the process by which nuclear interactions occur. The nuclear structure of hydrogen and helium is examined and then the precise step by step process is shown by which all isotopes of the known elements are constructed. Physical models are offered to depict the structure of the most common isotope of each element.